
SOLVED: Title: Calculation of Amount of NaOH for 1N Solution Text: 1N NaOH Solution Homework Why have you used 4.5 g of NaOH to prepare 100 mL of 1N NaOH solution?

100 mL of 0.1 N NaOH solution is required neutralization of 0.49 g of a dibasic acid. What is the molecular weight of the acid? (a) 49 (b) 98 (c) 490 (d) 196

50 ml of 1 N NaOH is mixed with 150 ml of 2 NCa(OH)2. The molarity of OH ions in the resultingsolution will - Brainly.in

How to prepare 0.1 N NaOH solution | 250 ml of 0.1 N NaOH solution | 0.1 N NaOH solution calculation - YouTube

61. The volume of 0.1 M NaOH required to neutralize 10 mL of 0.1 M HCI (A) 10 mL (B) 20 mL (C) 5 mL (D) 15 mL 000 Sa Id=18 almal hy weight is





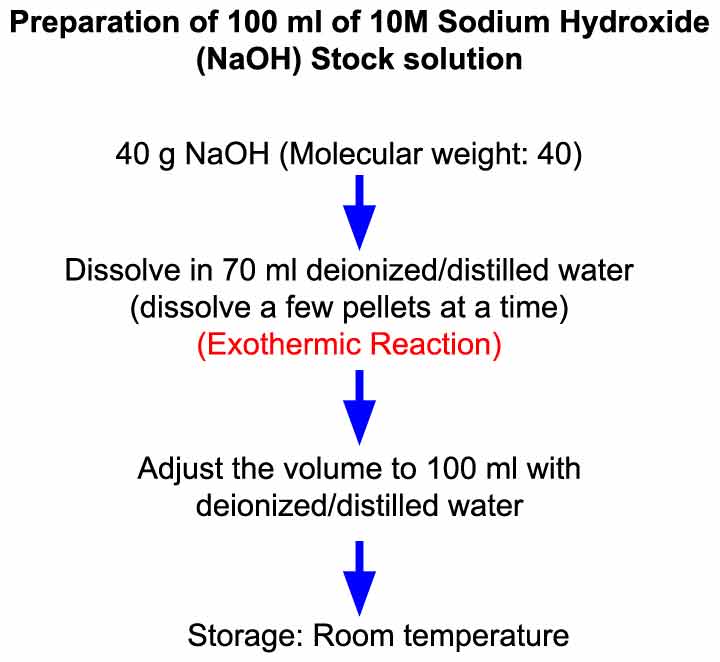

:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)








